Leading the FightAgainst Solid Tumors
About Us
Pioneering Novel Approaches
to Deliver on the Promise of TILs
At Turnstone, our mission is to develop new medicines to treat and cure patients with solid tumors.
Solid tumors present a major burden to society, with high mortality and poor outcomes associated with more advanced disease. Approved immunotherapies represent a significant advancement in the treatment of solid tumors, but many patients either do not respond or experience relapsed disease following an initial response. We believe the most significant challenge to creating curative immunotherapies in these patients is the low numbers of T cells that can recognize and attack the tumor, which we refer to as tumor‑reactive T cells.
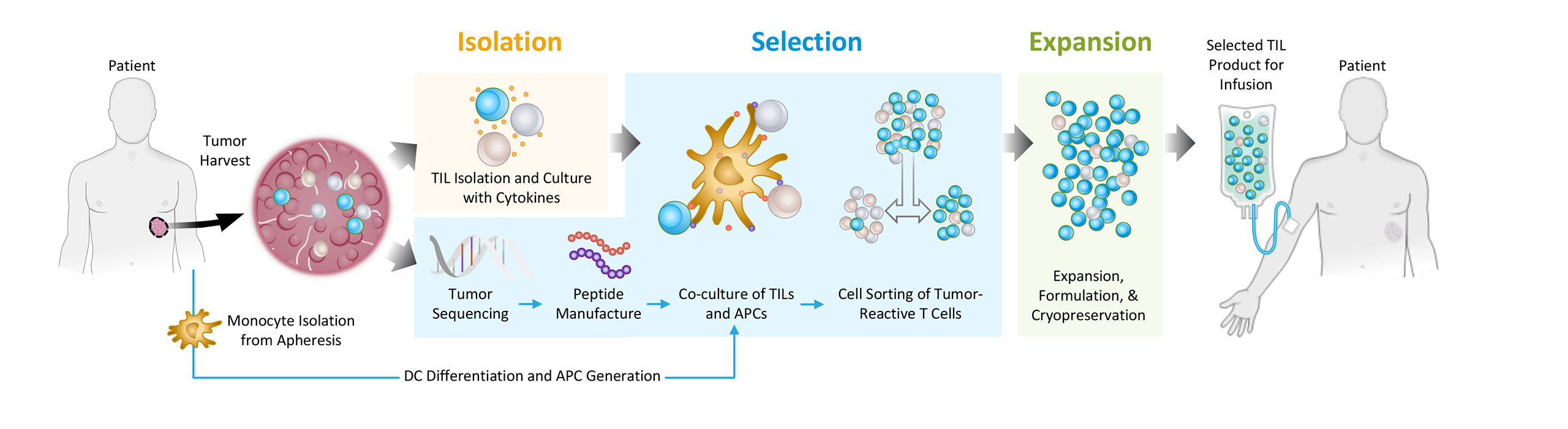
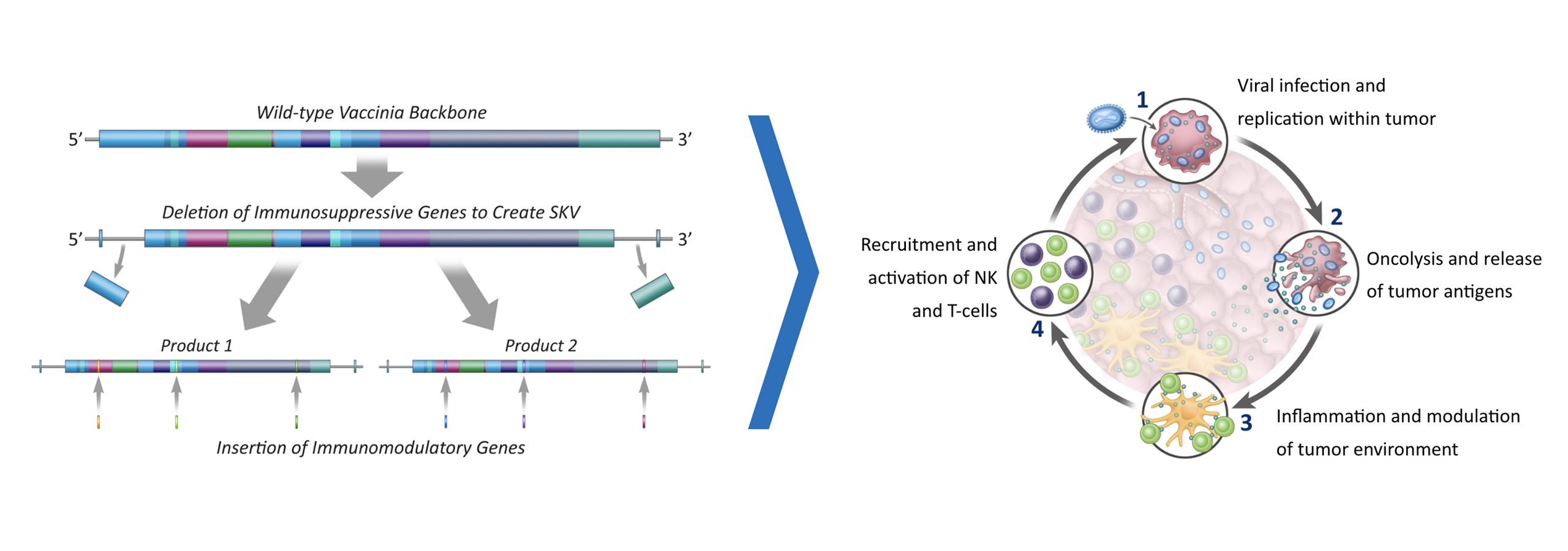
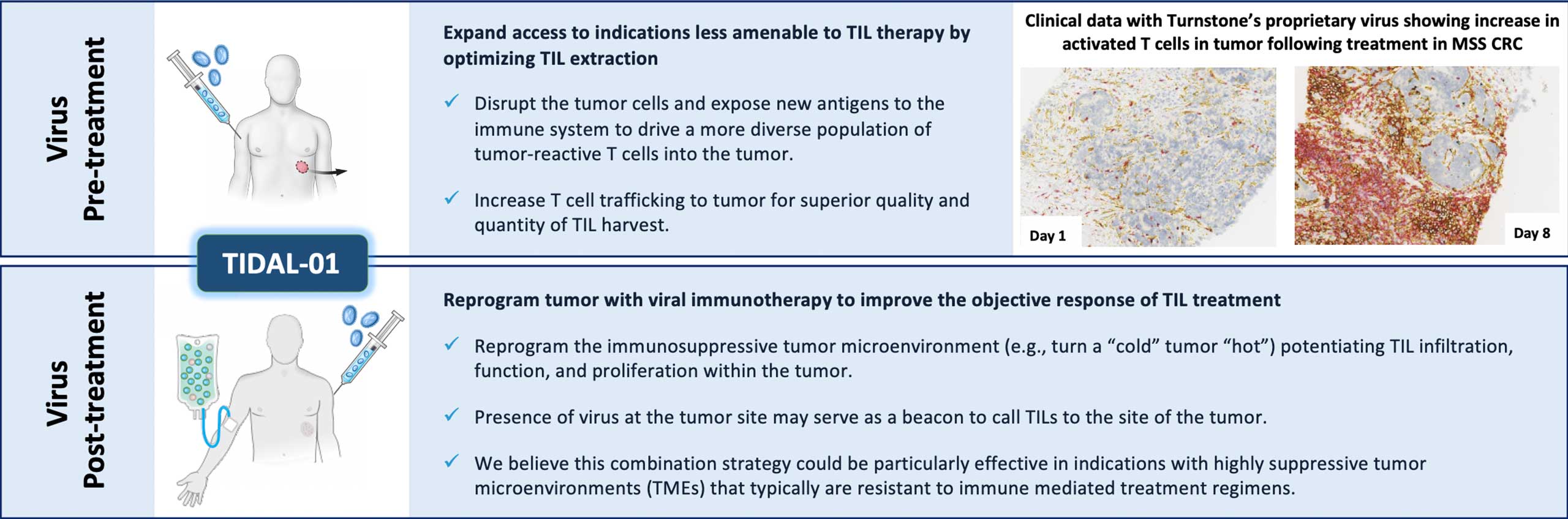
To address this problem, we are pioneering a differentiated approach to tumor infiltrating lymphocytes, or TILs. We are developing next-generation TIL therapies by selecting the most potent (meaning able to mediate an anti-tumor response) and tumor-reactive T cells, which we refer to as Selected TILs. We are developing next-generation TIL therapies for potential treatment across multiple solid tumors.
-
One Turnstone
-
One Focus
-

One Fight
Leadership
Experienced Team of
Leaders and Innovators
Turnstone has assembled a seasoned management team, an accomplished Board of Directors and a distinguished Scientific Advisory Board whose contributions to science have meaningfully advanced the field and helped inform our understanding of the relationship between cancer and the immune system.
Our passionate team of leaders comprises world-renowned professionals with deep expertise in TILs, cell therapy, tumor immunology, innate and adaptive immunity, oncolytic viruses, and in the discovery and development, manufacturing, and business and commercial development of complex biologics.

Sammy Farah, PhD, MBA
President & Chief Executive Officer

Saryah Azmat
Chief Operating Officer

Wendy Worcester, CPA
VP, Principal Finance and Accounting Officer

David Stojdl, PhD
SVP, Discovery Research

Chad Green, PhD
SVP, Technical Operations

TJ Langer
SVP, Cell Therapy Development and External Innovation

Adina Pelusio
SVP, Clinical Operations

Karen Major
VP, Regulatory

George Smith, MBA, PhD
VP, Cell Therapy Business Operations

Jerel Davis, PhD
Chairman & Director
Versant Ventures

Mike Burgess, MBChB, PhD
Executive Director
Turnstone Biologics

Sammy Farah, PhD, MBA
Director
Turnstone Biologics

Robert Gould, PhD
Director
Fulcrum Therapeutics

Rishi Gupta, JD
Director
OrbiMed

Kanya Rajangam, MD, PhD
Director
Senti Biosciences

William Waddill
Director
Former CFO Calithera Biosciences, OncoMed, and Ilypsa

Jeff Courtney
In Memoriam
Our Approach
Scientific Resources
Explore academic selection strategies that have demonstrated clinical proof of concept, relevant to Turnstone’s differentiated approach to TIL therapy:
Learn more about our scientific and clinical research, and the potential of Turnstone’s Selected TIL therapy in a broad range of solid tumors: